Our life -saving device for small babies is stuck in development | Medical Research
We have found the idea of Alexander Masters for a long time (we have found the idea of “outside the box ılan which failed because of the lack of financing in many life -saving drugs. But there is a solution: hopeless rich people, 11 March) interesting. And Prof Roger Bayston’s innovative devices with commercial production, regulatory barriers and their views on the problems of putting clinical use (Letters, 17 March).
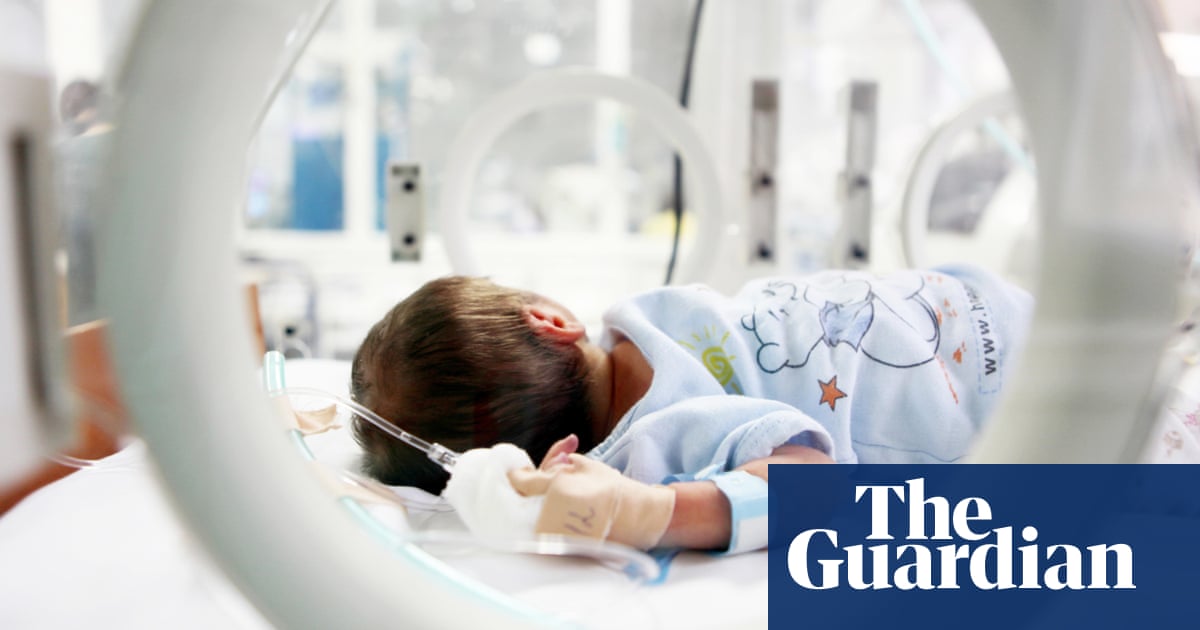
These problems are combined when the new device aims to develop a small subset of a rare disease or a more common problem, aiming to develop a hemodialysis device to treat small babies. This device was invented as a response to the parental pressure to “do something ğinde when newcastle infant dialysis and ultrafiltration system (NIDUS), newborn babies (usually for congenital abnormalities such as abdominal or heart conditions), renal failure and need hemodialysis to keep themselves alive.
This is a field of clinical need for infants that require dialysis but cannot be treated with peritoneal dialysis, and do not have hemodialysis devices to do this safely in the UK. Therefore, despite the warnings of the manufacturers and the potential serious problems known to do so, alternatives are designed for adults and children over 8 kg and licensed machines are used. There are no registration of the devices used in unmissable and there are no problems.
The new NIDUS device has been developed for more than 20 years from manual circuits from various prototypes to the existing device, with the time, expertise and skills of NHS Medical Engineering, Nursing and Health Personnel. It has been subjected to a successful clinical research financed by the Institute of National Health and Care Research (ie the taxpayer). However, it is now trapped, deprived of relevant expertise and funds to go through regulatory approvals. Nobody is limited to what they can spend from this device to help develop manufacturing companies.
Although there are paths for “orphan” drugs used in rare diseases in the UK, there is no such as “orphan” devices like this. We need clearly regulating systems for patient safety, but by ways for groundbreaking technology.
Dr Heather Lambert and Dr Malcolm Culthard
Retired pediatric Nefrologists, Newcastle Upon Tyne




